Evaluation of Barium Titanate Polymorphs by Rietveld Analysis
Introduction
Barium titanate (BaTiO₃) is a representative ferroelectric material, used in capacitors and electronic components such as pyroelectrics and piezoelectrics. Barium titanate exists as tetragonal and cubic systems at room temperature. The mass fractions of these two phases affect its permittivity. In addition, the c/a ratio of the tetragonal phase also affects permittivity. Therefore, it is important to determine the mass fractions of the polymorphs and lattice constants to control the permittivity of barium titanate. Rietveld analysis allows you to easily calculate mass fractions and lattice constants based on the X-ray diffraction pattern. In this example, the X-ray diffraction pattern was obtained using a compact X-ray diffractometer, equipped with the high-resolution and high-speed one-dimensional detector D/teX Ultra250. We evaluated mass fractions and lattice constants of barium titanate by Rietveld analysis.
Measurements and results
We used a commercially available barium titanate powder (FUJIFILM Wako Pure Chemical Corporation) for the measurement. Rietveld analysis was performed using integrated X-ray analysis software SmartLab Studio II. Results of the Rietveld analysis are shown in Figure 1, Table 1 and 2. Mass fractions (Table 1) and lattice constants (Table 2) can be obtained simultaneously in a single Rietveld analysis. Additionally, it is possible to easily analyze multiple data sets all at once using an analysis template.
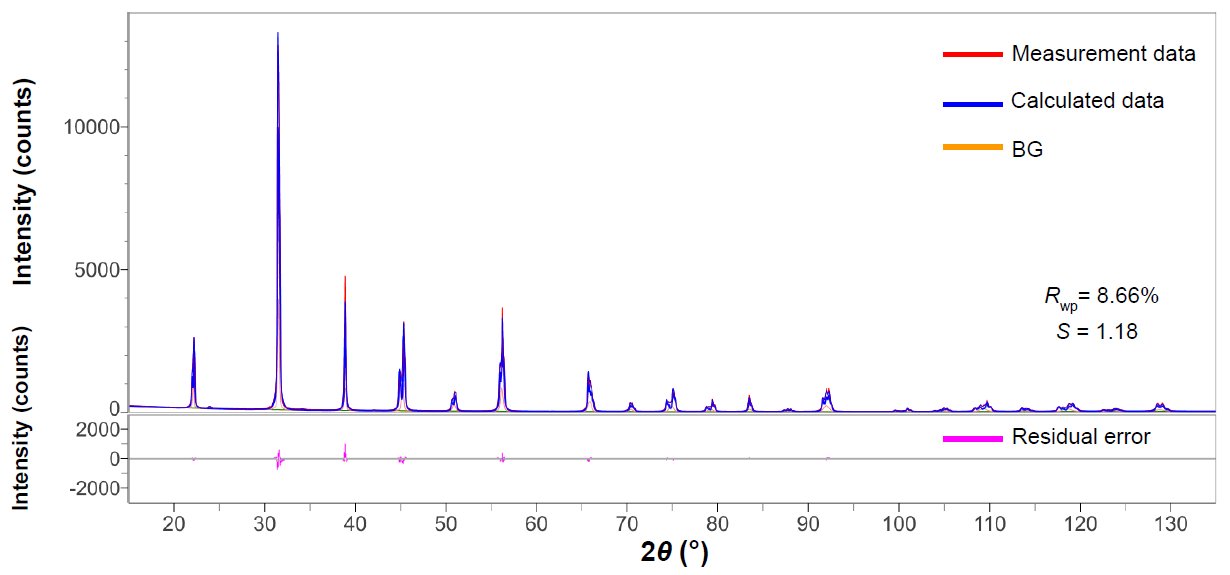
Figure 1: Profile fitting results using Rietveld analysis for the barium titanate sample
Table 1: Quantitative results of the barium titanate powder sample
| Crystal phase | Mass fraction (mass%) |
| BaTiO₃ (tetragonal) | 76.1 |
| BaTiO₃ (cubic) | 22.8 |
| BaCO₃ | 1.12 |
Table 2: Lattice constants and the c/a ratio of the tetragonal barium titanate
| a (Å) | c (Å) | c/a ratio |
| 3.99525 | 4.03494 | 1.009934 |

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.
