Background
If polymorphic impurities, degradation products and anything else other than the desired crystalline forms are present in a raw drug substance of a pharmaceutical product, they might affect the stability, functionality and effectiveness of the pharmaceutical product, so increasingly strict control of impurities is required.
Investigation
Powder X-ray diffractometry is a powerful analysis technique. Conventionally, to detect trace impurities of ≤1%, a rotating anode X-ray generator was required as a powerful X-ray source. With Rigaku's newly developed high-speed one-dimensional detector, it becomes possible to analyze such impurities in a short time at high sensitivity even if the detector is installed with a 2 kW sealed tube X-ray source unit. Figure 1 shows an X-ray diffraction pattern taken with Rigaku's Ultima IV multipurpose diffraction system when the 0.2 wt% Type II as polymorphic impurities is added to tolbutamide (a drug for diabetes) Type I. The presence of Type II can be confirmed at the 10.3° peak under these measurement conditions: X-ray source: Cu K-alpha 40 kV 50 mA, focusing X-ray optics, and scan speed: 2°/min.
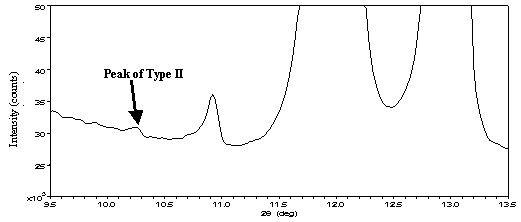
Figure 2 shows the calibration curve when the contained amounts of Type II are varied within the range of 0.2 to 5 wt%. This figure shows that a good linearity is obtained.

