nano3DX
True submicron CT scanner with contrast-enhancing X-ray anode
3D X-ray microscope
Rigaku nano3DX is a true submicron resolution CT (computed tomography) scanner. The parallel beam geometry combined with an ultrabright 1200 W rotating anode X-ray source enhances the contrast of soft materials, which are normally difficult to image using high-energy X-ray sources. The X-ray anode can be selected from Cr (5.4 keV), Cu (8 keV), or Mo (17 keV) for low-energy and pseudo-monochromatic radiation to maximize the contrast for the given sample material and size. With the highest magnification lens, the nano3DX can achieve 325 nm voxel resolution and true submicron (700 nm) spatial resolution.


nano3DX Overview
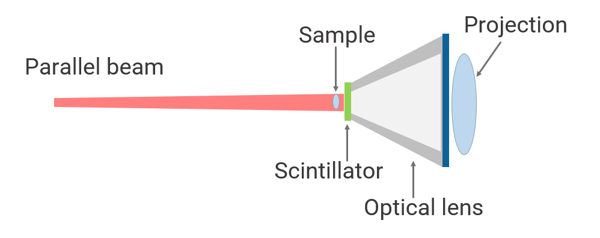
How do I achieve high resolution?
Rigaku nano3DX uses parallel beam geometry. This geometry uses an optical lens to magnify the sample image. It does not use the X-ray beam divergence and eliminates blurring caused by the X-ray focus size and drift. With the 20X magnification lens, you can achieve 325 nm voxel resolution and true submicron (700 nm) spatial resolution.
At this resolution, you can see individual carbon fibers (~7.5 microns), the intricate structures of seeds, small insects, etc.
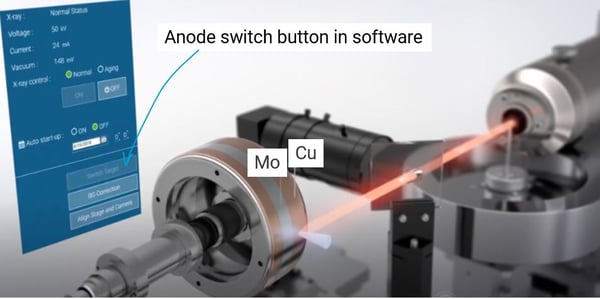
How do I change X-ray anodes?
Rigaku nano3DX is equipped with a dual-wavelength rotating anode X-ray generator, MicroMax-007 HF. The rotating X-ray anode is made of Cu, providing Cu characteristic radiation of 8 keV. Another anode material, Cr 5.4 keV, Mo 17 keV, or W for Bremsstrahlung radiation, can be added to the Cu anode to provide the second radiation. You can switch between two radiations with a simple click on the instrument control software.
You can image an even 5% density difference in organic materials by using optimized radiation and differentiate amorphous and crystalline phases, high- and low-density polyethylene, etc.
Do I need to prepare my sample in a special way?
No, X-ray CT measurements require minimum to no sample preparation. As far as the sample size is close to the FOV (field of view), you can mount the sample on the sample stage with a small pin as is, and you are ready to run a CT scan.
Because X-ray CT experiments are done in the air and do not require conductivity on the sample as is the case for SEM (scanning electron microscopy), organic samples such as plants can be measured without a drying or coating process.

nano3DX Features
nano3DX Videos
nano3DX Specifications
| Voxel resolution | 325 nm — 10 μm | |
|---|---|---|
| Field of view (FOV) | 0.66 — 20 mm | |
| Field of view (FOV) 0.66 — 20 mm Maximum sample size | 20 mm diameter x 40 mm height | |
| Speed (shortest scan time) | 30 sec | |
| Geometry | Parallel beam geometry | |
| X-ray source | 1200 W rotating anode microsource | |
| X-ray energy | Pseudo monochromatic sources: Cr (5.4 keV), Cu (8 keV), Mo (17 keV) | Traditional source: W operated at 60 kV |
|
| Lenses | 1.25X, 2.5X, 5X, 10X, 20X | |
| Detector | High-resolution sCMOS | |
| Detector pixel size | 6.5 microns | |
| Detector size | 2048 x 2048 pixels | |
| Dimensions | 1300 (W) x 1880 (H) x 655(D) mm (PC, chiller, vacuum pump not included) | |
| Weight | Approx. 600 kg | |
nano3DX Options
nano3DX Application Notes
The following application notes are relevant to this product
-
B-XRI1035 - Ceramic Additive Manufacturing
-
B-XRI1017 - Analysis of volume fraction and thickness of bran layer using a high-resolution 3D X-ray microscope
-
BATT1023 - Assessment of Particle Diameter and Interparticle Voids for Cathode Material for Lithium-Ion Batteries
-
BATT0005 - Porosity Analysis of Cathode-Coated Sheet
-
BATT0003 - Battery Performance
-
RACCT9045 - Tablet Crystallinity Analysis by X-ray CT
-
RACCT9042 - Microparticle Coating Analysis by X-ray CT
-
RACCT9041 - Famotidine Tablet Comparison by X-ray CT
-
RACCT9040 - Degradation of Sustained-release Dosage Tablet Imaged by X-ray CT
-
RACCT9038 - Brand Name vs Generic Atorvastatin Tablets Comparison by X-ray CT
-
RACCT9037 - Aspirin Tablet Coating Delamination Imaging by X-ray CT
-
RACCT9009 - Chocolate Candy Coating Analysis by X-ray CT
-
RACCT9005 - Shoe Sole Compression and Pore Size Analysis by X-ray CT
-
RACCT9003 - Makeup Sponge Wear and Tear Analysis by X-ray CT
-
RACCT9002 - Insulator Porosity and Cell Wall Thickness Analysis by X-ray CT
-
RACCT9001 - Earplug Pore Size Analysis by X-ray CT
-
RACCT9000 - CFRP Void and Fiber Analysis by X-ray CT
-
B-XRI1007 - Visualization of Components in Ceramic Composites using a High-resolution 3D X-ray Microscope
-
B-XRI1004 - Visualization and Size Analysis of Foreign Matter in CFRP using a High-resolution 3D X-ray Microscope
-
XCT2101 - Textile and Fiber Industry using the nano3DX
-
XCT2102 - Submicron CT for the Pharmaceutical Industry
-
XCT2201 - Submicron CT for the Life Sciences: Animal and Tissue Research Applications
-
XCT2103 - Submicron CT for Plant Research Applications
-
B-XRI1002 - Observation of Milk Protein Aggregation in String Cheese
-
B-XRI1001 - CT Observation of a Laminated Battery Cell by X-ray Microscopy
nano3DX Resources
Webinars
- High-resolution CT Data Collection Techniques Part 1
- High-resolution CT Data Collection Techniques Part 2
Blog articles
- How Much Does a Micro CT Scanner Cost?
- 7 Common Problems with X-ray CT & How To Avoid Them
- CT vs. SEM: Which Is Better?
Rigaku Journal articles
![]() Basics of X-ray CT Reconstruction--Principles and Applications of Iterative Reconstruction
Basics of X-ray CT Reconstruction--Principles and Applications of Iterative Reconstruction ![]() Application of the nano3DX X-ray microscope to biological specimens
Application of the nano3DX X-ray microscope to biological specimens ![]() Examination of electronic components with the nano3DX X-ray CT microscope
Examination of electronic components with the nano3DX X-ray CT microscope![]() In situ microscopic structural investigations with a three-dimensional X-ray microscope: nano3DX
In situ microscopic structural investigations with a three-dimensional X-ray microscope: nano3DX![]() A primer on the use of the nano3DX high-resolution X-ray microscope
A primer on the use of the nano3DX high-resolution X-ray microscope![]() Visualization and analysis of pharmaceutical solids by X-ray microscopy
Visualization and analysis of pharmaceutical solids by X-ray microscopy
Publications
Visit the Publication Library to access articles relevant to nano3DX
Other resources
nano3DX Events
Learn more about our products at these events
-
EventDatesLocationEvent website
-
Pittcon 2026March 9 2026 - March 11 2026San Antonio, TX, USA
nano3DX
Testimonials
-

The technical knowledge, professionalism, willingness to answer ours question in a timely manner, is appreciated. My users also enjoy the educational webinars offered on a wide range of imaging subjects.
Read the full testimonialGerald PoirierDirectorAdvanced Materials Characterization Lab | University of Delaware -

Very pleasant experience with CT Lab GX 130 and nano3DX. Excellent build quality. Very knowledgeable staff and professional service.
Read the full testimonialLeilei Yin, PhDRetiredThis testimonial was given by Dr. Yin when he was employed at The Beckman Institute University of Illinois at Urbana-Champaign.

Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.


