XtaLAB Synergy-S
Benefits
- Fast, accurate data collection due to high-speed kappa goniometer, high-flux X-ray source, fast, low-noise X-ray detector, and highly optimized instrument control software.
- Enhanced experimental versatility when the dual-source option is selected from three possible wavelengths (Mo, Cu, or Ag).
- Highest level of user safety with multiply redundant electromechanical safety circuits built into the ergonomically designed radiation enclosure.
- Minimize your downtime by utilizing built-in online diagnostics and troubleshooting to diagnose and fix almost all problems without a site visit.
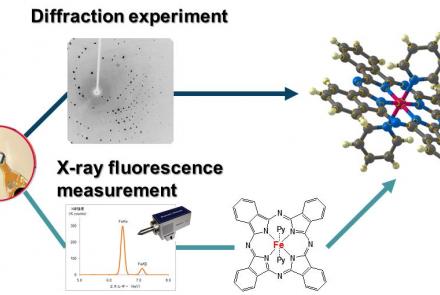
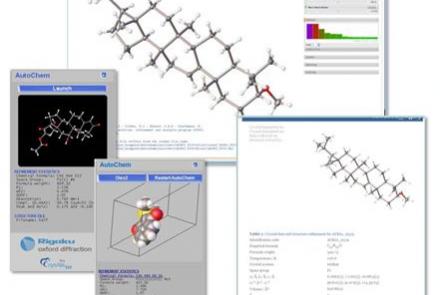
- Automatically solve structures and determine what your sample is in a few seconds before committing to a full dataset by using the “What is this?” feature.
- Improve your ability to investigate small samples because the solid state pixel array technology of the HyPix X-ray detectors means that X-ray photons are counted instantaneously as they arrive at the detector. There is no conversion to visible light by a scintillator so the energy of the photon can be assessed at moment of detection leading to essentially noise free images. And noise-free images means you can count longer for weakly diffracting crystals without a loss in data quality arising from detector noise.
- Optimize data collection speed when you select the optional HyPix-Arc 100° or HyPix-Arc 150° curved detectors, which allow theta coverage exceeding the largest detectors while still offering the highest-performing detection technology.
- Enhance your ability to resolve large unit cells, twins or incommensurate lattices when you select the optional motorized variable beam slit in order to alter divergence to adapt the source to your sample’s requirements.
Read what our customers say about the XtaLAB Synergy-S
Download the brochure
To download this brochure, please fill out the following form (Fields marked with * are required)
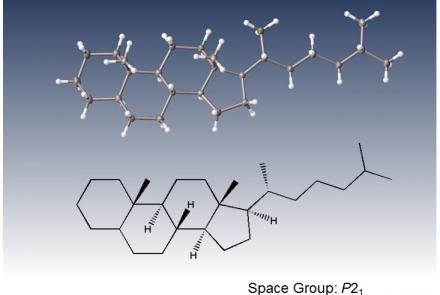
Single or dual microfocus X-ray diffractometer for all your crystallography needs
A fast and agile single crystal X-ray diffractometer for small molecule 3D structure analysis
Specifications and features
| Product name | XtaLAB Synergy-S |
| Core attributes | Single or dual microfocus sealed tube X-ray source diffractometer with hybrid pixel array detector and kappa goniometer |
| Detectors | HyPix-6000HE or optionally the large theta coverage detectors HyPix-Arc 100° or HyPix-Arc 150° |
| X-ray source | PhotonJet-S X-ray source with new microfocus sealed tube that incorporates a new mirror design and new alignment hardware. Three target types are available (Mo, Cu, Ag). |
| Goniometer | Fast kappa geometry goniometer that allows data collection scan speeds of up to 10°/sec. |
| Accessories | Oxford Cryostream 1000, Oxford Cobra, XtaLAB Synergy FLOW robotic system, XtalCheck-S, High Pressure Kit. |
| Computer | External PC, MS Windows® OS |
| Core dimensions | 1300 (W) x 1875 (H) x 850 (D) mm |
| Mass | 550 kg (core unit) |
| Power requirements | 1Ø, 90-130V 15A or 180-260V 4A |
Options and Accessories
The following accessories are available for this productApplication Notes
The following application notes are relevant to this productApplication Bytes
Learn more about our products at these events
| Booth number | Date | Location | Event website | |
|---|---|---|---|---|
| Webinar: Illuminating The World of Sub-Micron Crystal Structures with the XtaLAB Synergy-ED: A Review | Webinar | Register Now | ||
| UKPorMat | - | Liverpool, United Kingdom | Website | |
| 30th Croatian-Slovenian Crystallographic Meeting | - | Veli Losinj, Croatia | Website | |
| Zurich School of Crystallography | - | Zurich, Switzerland | Website | |
| Konwersatorium Krystalograficzne | - | Wrocław, Poland | Website | |
| AFC 2024 (French Cristallography Meeting) | - | Montpelier, France | Website | |
| ACA 2024 | - | Denver, CO | Website | |
| ECM2024 | - | Padova, Italy | Website | |
| Epdic 18 | - | Padova, Italy | Website | |
| 11th Meeting of the Young Crystallographers at Rigaku | - | Neu-Isenburg, Germany | Learn more | |
| Single-Crystal Users’ Meeting | - | Neu-Isenburg, Germany | Learn more |