Our Mission
To contribute to the enhancement of humanity through scientific and technological development


Because we understand
materials analysis can be
daunting, we provide
easy-to-use tools to support
you in getting the results
and insight you need with
confidence.
Our clients and awards
The industries we serve
Our X-ray, electron, Raman, and thermal analysis instruments serve in a wide range of industries to help you analyze various materials for both R&D and process control.
Batteries
X-ray analyses are ideal for a wide range of battery materials analyses, from atomic-level structural changes in electrodes during the charging and discharging process to packaged battery failure analysis.
Learn more >Semiconductor
X-ray metrology is widely used for elemental analysis, layer structure analysis, and dislocation mapping, supporting the semiconductor industry in research and development, process optimization, and quality control.
Learn more >
Electronics
X-ray analyses play an essential role in the development and process optimization of electronics by providing elemental and structural analyses as well as imaging inspections for failure analysis.
Learn more >
Cement
X-ray analyses reveal the detailed characteristics of raw materials and cement products, contributing to process optimization and quality control.
Learn more >
Life science
X-ray and electron diffractometers are essential in life science for solving protein and small molecule structures at the early stages of research and development. Multi-modal molecular imaging is used for pre-clinical studies, and X-ray CT for soft tissue and plant imaging.
Learn more >
Pharmaceuticals
X-ray and electron diffraction solve crystal structures early in the drug development process; powder X-ray diffraction provides polymorphs and crystallinity quantification later in the later stage. Raman spectroscopy is used for raw materials and counterfeit identification.
Learn more >Our products
We have been designing and providing various analytical instruments for over 70 years, always striving to make them easy to use and make your life as easy as possible.
Semiconductor metrology
X-ray metrology tools for semiconductor process R&D and high-volume manufacturing.
Learn more >
Crystallography
X-ray and electron diffractometers for crystallography to determine the three-dimensional structure of crystalline materials at the atomic level.
Learn more >
X-ray diffraction & scattering
X-ray diffractometers for studying the crystalline and amorphous materials at the atomic level and X-ray scattering instruments for investigating nanometer-scale morphologies.
Learn more >
XRF Spectrometers
X-ray fluorescence spectrometers for qualitative and quantitative elemental analysis of various materials, including minerals, metals, cement, petroleum products, and polymers.
Learn more >
X-ray imaging & NDT
Radiography-based NDT systems for product inspection and CT scanners for materials and life science research, process optimization, and failure analysis.
Learn more >
Raman spectroscopy
Handheld Raman analyzers for chemical threat detection and identification and verification of various materials, including drugs and explosives.
Learn more >Let's learn together
Learning a new analytical technique can be overwhelming. We are here to help you with all the educational events, content, and resources.

Webinars
Watch on-demand webinars or join us for the next live webinars to learn new analysis techniques and industry trends.
Find webinars >
Application library
Explore analysis examples by products, industries, or analysis techniques and see how each analytical technique can be applied to your area of interest.
Explore applications >
Rigaku Journal
The Rigaku Journal is a scientific and technical journal published by Rigaku to serve the X-ray analysis community, covering a wide range of X-ray diffraction (XRD), X-ray fluorescence (XRF), X-ray imaging, and other analytical applications.
Browse Rigaku Journal articles >
Blogs
Through Rigaku blog articles, we strive to answer questions our customers frequently ask us, offer solutions to common problems, and share the latest news and developments in the world of analytical instruments.
Explore Rigaku blogs >
Publications library
See how people use our products for their research in published journal articles.
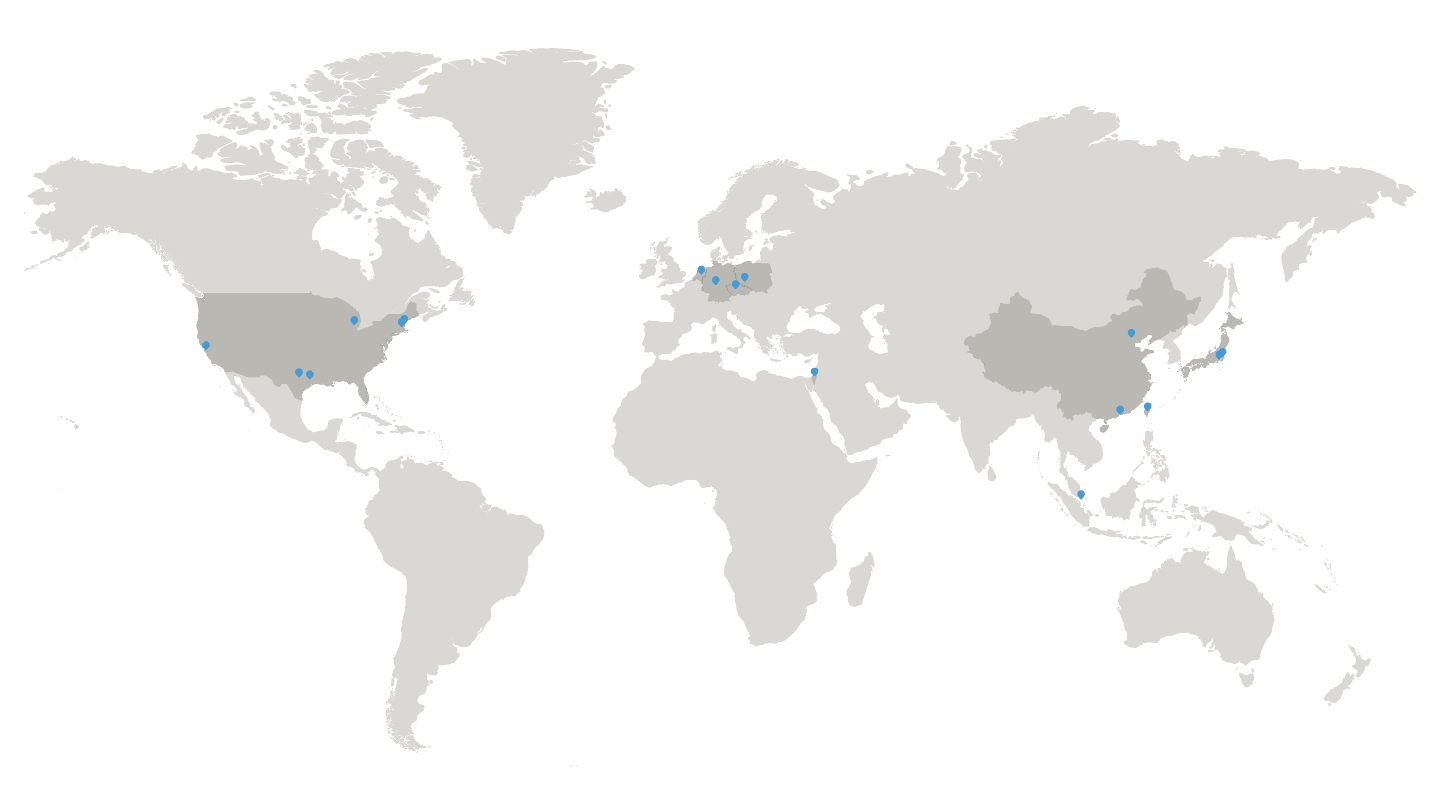
Search publications >Where we work
You can find our local offices and representatives globally. You can also find us at conferences and tradeshows around the world.
Locations Find Rigaku offices and local partners
Find the local Rigaku representatives for sales, applications, or technical services.

Events Join us at the next event
Are you going to a conference or tradeshow soon? These are great opportunities to speak with our experts and find the best fit for your research and process control analytical needs.


Contact Us
Whether you're interested in getting a quote, want a demo, need technical support, or simply have a question, we're here to help.