|
Monthly message
At the end of April, Rigaku finalized the acquisition of Agilent’s XRD business. Our combined single crystal business of small molecule and macromolecular solutions will now operate as a single business unit named Rigaku Oxford Diffraction.

Rigaku Oxford Diffraction’s European R&D and manufacturing
facility is located in beautiful Wroclaw, Poland
Going forward, Crystallography Times will cover all topics of chemical and macromolecular crystallography. While the two fields are still separated in terms of the underlying materials being studied, there is definitely a movement in the direction of a more common set of instrumentation requirements. The interest in large chemical entities, such as MOFs, is pushing the need of the chemical crystallographer in the direction of higher performance instrumentation, while the existence of a large number of PX beamlines has transformed many PX home labs into screening facilities. As crystallography continues to evolve in the years ahead, Rigaku Oxford Diffraction will dedicate our efforts to providing the best instrumentation for your research needs.
Crystallography
in the news
May 1, 2015. Researchers from Caltech and Heidelberg University have combined their expertise to track a ribosomal protein in yeast all the way from its synthesis in the cytoplasm to its incorporation into a developing ribosome within the nucleus. In so doing, they have identified a new chaperone protein, known as Acl4, that ushers a specific ribosomal protein through the construction process and a new regulatory mechanism that likely occurs in all eukaryotic cells.
May 5, 2015. Alexander Rich Dies at 90. Dr. Rich employed X-ray diffraction to confirm DNA's double helix in 1973. For nearly six decades, Dr. Rich doggedly investigated DNA and RNA. He co-founded several biotechnology companies, including Repligen in 1981 and Alkermes in 1987.
May 5, 2015. A team led by Stanford scientists has created software that tackles the big data problem for single crystal X-ray diffraction experiments at the SLAC National Accelerator Laboratory. The program allows researchers to tease out more details while using far fewer samples and less data and time. The tool works by analyzing partial X-ray diffraction data that might otherwise be discarded and comparing them with known data to fill in the blanks. When applied to a complete set of data, this can reveal new structural details.
May 13, 2015. A team of researchers from the US National Cancer Institute and the National Heart, Lung, and Blood Institute has developed an enhanced version of a cryo-electron microscope, meaning that these powerful tools for determining protein structures are more powerful than ever.
May 14, 2015. Michael Rossmann, Purdue University's Hanley Distinguished Professor of Biological Sciences, led a team of researchers who discovered the structure of a key, enzyme-like protein called R135, which is contained in fibers on the outer surface of an unusually large virus called the mimivirus. The structure of R135 is similar to an enzyme called aryl alcohol oxidase, which is found in a fungus and is involved in biodegrading lignin in plant cell walls.
May 15, 2015. An electron crystallography technique developed by RIKEN and the University of Tokyo makes it possible to probe hidden details of small three-dimensional protein crystals as a potent new tool for studying the function and structures of biological macromolecules
May 26, 2015. At the Advanced Light Source (ALS), researchers have created an operational mode for synchrotron light sources that provides full control of the timing and repetition rate of single X-ray pulses without affecting beams for other users. The mode, which is called "pseudo-single-bunch kick-and-cancel," or PSB-KAC for short, works by displacing and routing a single electron bunch—dubbed the "camshaft bunch"—from the multi-bunch train of an electron beam in a synchrotron storage ring so that only X-ray light from this camshaft bunch reaches the experiment.
May 26, 2015. Scientists from Japan report that their revolutionary crystallographic technique has determined the stereochemistries of molecules with axial and planar chiralities, where classical methods had failed. This team, led by Makoto Fujita from the University of Tokyo, captured the chemistry community's attention just a few years ago when they reported a crystallographic technique capable of analyzing compounds without first needing to crystallize them.
Product spotlight: SuperNova
Single or dual microfocus X-ray source diffractometer
The SuperNova from Rigaku Oxford Diffraction combines hi-flux microfocus sources with the choice of either our patented fast, self-optimizing S2 CCD detectors or a Pilatus HPAD detector.
The combination of high intensity and fast detectors enables rapid data collection, with easy software-switching between molybdenum and copper wavelengths (from the inventors of dual source X-ray diffractometers). The SuperNova is compact and fully integrated, and as a highly automated dual source system needs little servicing to maximize uptime and throughput. It is the ideal diffractometer for small molecule crystallography in research laboratories and leading analytical service facilities, as well as facilities that want the flexibility to offer macromolecular data collection capabilities in addition to small molecule capabilities.

Visit Rigaku.com for more information about the SuperNova.
Lab spotlight: Scheer Research Group
Prof. Dr. Manfred Scheer
Institute of Inorganic Chemistry
University of Regensburg
Germany

Prof. Scheer’s group is active in the field of molecular chemistry, with a focus on a materials scientific utilization of the synthesized compounds. Their research is directed towards the chemistry of substituent-free main group element ligand complexes, mainly of the elements of group 15. Furthermore, nanoscale fullerene-like clusters are prepared starting from pentaphosphaferrocene and Cu(I) halides, and they are interested in their supramolecular and capsule chemistry. Such compounds are formed in template-controlled reactions as a consequence of molecular recognition.


Useful link: LigSearch
The LigSearch server predicts ligands that might bind to a given protein target. Its chief aim is to help experimentalists who are planning to solve a specific protein structure to identify small molecules that might form a complex with the protein. The server uses resources such as ChEMBL, ChEBI and the structures in the PDB to find potential ligands.
Janet Thorton’s group has developed and maintains a number of freely available databases and tools, many in collaboration with researchers at other institutes. Some of the resources were developed during the Thornton Group's time at University College London. A complete list can be found here.
Selected
recent crystallographic papers
Solution Structure of the Atg1 Complex: Implications for the Architecture of the Phagophore Assembly Site. Köfinger, Jürgen; Ragusa, Michael J.; Lee, Il-Hyung; Hummer, Gerhard; Hurley, James H. Structure. May2015, Vol. 23 Issue 5, p809-818. 10p. DOI: 10.1016/j.str.2015.02.012.
Absolute refinement of crystal structures by X-ray phase measurements. Morelhão, Sérgio L.; Amirkhanyan, Zohrab G.; Remédios, Cláudio M. R. Acta Crystallographica. Section A, Foundations & Advances. May2015, Vol. 71 Issue 3, p291-296. 6p. DOI: 10.1107/S2053273315002508.
Against the odds? De novo structure determination of a pilin with two cysteine residues by sulfur SAD. Gorgel, Manuela; Bøggild, Andreas; Ulstrup, Jakob Jensen; Weiss, Manfred S.; Müller, Uwe; Nissen, Poul; Boesen, Thomas. Acta Crystallographica: Section D. May2015, Vol. 71 Issue 5, p1095-1101. 7p. DOI: 10.1107/S1399004715003272.
Atomic-resolution structure of the a-galactosyl binding Lyophyllum decastes lectin reveals a new protein family found in both fungi and plants. van Eerde, André; Grahn, Elin M.; Winter, Harry C.; Goldstein, Irwin J.; Krengel, Ute. Glycobiology. May2015, Vol. 25 Issue 5, p492-501. 10p. DOI: 10.1093/glycob/cwu136.
Using support vector machines to improve elemental ion identification in macromolecular crystal structures. Morshed, Nader; Echols, Nathaniel; Adams, Paul D. Acta Crystallographica: Section D. May2015, Vol. 71 Issue 5, p1147-1158. 12p. DOI: 10.1107/S1399004715004241.
Solvation and cavity occupation in biomolecules. Lynch, Gillian C.; Perkyns, John S.; Nguyen, Bao Linh; Pettitt, B. Montgomery. BBA - General Subjects. May2015, Vol. 1850 Issue 5, p923-931. 9p. DOI: 10.1016/j.bbagen.2014.09.020.
Beyond crystallography: Diffractive imaging using coherent x-ray light sources. Jianwei Miao; Tetsuya Ishikawa; Robinson, Ian K.; Murnane, Margaret M. Science. 5/1/2015, Vol. 348 Issue 6234, p530-535. 6p. DOI: 10.1126/science.aaa1394.
The crystallography of correlated disorder. Keen, David A.; Goodwin, Andrew L. Nature. 5/21/2015, Vol. 521 Issue 7552, p303-309. 7p. DOI: 10.1038/nature14453.
The targeted recognition of Lactococcus lactis phages to their polysaccharide receptors. McCabe, Orla; Spinelli, Silvia; Farenc, Carine; Labbé, Myriam; Tremblay, Denise; Blangy, Stéphanie; Oscarson, Stefan; Moineau, Sylvain; Cambillau, Christian. Molecular Microbiology. May2015, Vol. 96 Issue 4, p875-886. 12p. DOI: 10.1111/mmi.12978.
Supramolecular self-assembly of nucleotide–metal coordination complexes: From simple molecules to nanomaterials. Zhou, Pei; Shi, Rufei; Yao, Jian-feng; Sheng, Chuan-fang; Li, Hui. Coordination Chemistry Reviews. May2015, Vol. 292, p107-143. 37p. DOI: 10.1016/j.ccr.2015.02.007.
X-domain of peptide synthetases recruits oxygenases crucial for glycopeptide biosynthesis. Haslinger, Kristina; Peschke, Madeleine; Brieke, Clara; Maximowitsch, Egle; Cryle, Max J. Nature. 5/7/2015, Vol. 521 Issue 7550, p105-109. 5p. 7 Diagrams, 1 Chart, 7 Graphs. DOI: 10.1038/nature14141.
Bismuth–lithium bonding in the ion pairs: LiBiL2, where L = a porphyrin or a salen ligand. Balasanthiran, Vagulejan; Chisholm, Malcolm H.; Durr, Christopher B. Dalton Transactions. 5/7/2015, Vol. 44 Issue 17, p8205-8213. 9p. DOI: 10.1039/c5dt00256g.
Determination of Hydrogen Bond Structure in Water versus Aprotic Environments To Test the Relationship Between Length and Stability. Sigala, Paul A.; Ruben, Eliza A.; Liu, Corey W.; Piccoli, Paula M. B.; Hohenstein, Edward G.; Martínez, Todd J.; Schultz, Arthur J.; Herschlag, Daniel. Journal of the American Chemical Society. 5/6/2015, Vol. 137 Issue 17, p5730-5740. 11p. DOI: 10.1021/ja512980h.
A linker strategy for the production and crystallization of Toll/interleukin-1 receptor/resistance protein domain complexes. Williams, Simon J.; Ve, Thomas; Kobe, Bostjan. PEDS: Protein Engineering, Design & Selection. May2015, Vol. 28 Issue 5, p137-145. 9p. DOI: 10.1093/protein/gzv013.
Effects of self-seeding and crystal post-selection on the quality of Monte Carlo-integrated SFX data. Barends, Thomas; White, Thomas A.; Barty, Anton; Foucar, Lutz; Messerschmidt, Marc; Alonso-Mori, Roberto; Botha, Sabine; Chapman, Henry; Doak, R. Bruce; Galli, Lorenzo; Gati, Cornelius; Gutmann, Matthias; Koglin, Jason; Markvardsen, Anders; Nass, Karol; Oberthur, Dominik; Shoeman, Robert L.; Schlichting, Ilme; Boutet, Sébastien. Journal of Synchrotron Radiation. May2015, Vol. 22 Issue 3, p644-652. 9p. DOI: 10.1107/S1600577515005184.
Nanometal Surface Energy Transfer Optical Ruler for Measuring a Human Telomere Structure. Armstrong, Rachel E.; Riskowski, Ryan A.; Strouse, Geoffrey F. Photochemistry & Photobiology. May2015, Vol. 91 Issue 3, p732-738. 7p. DOI: 10.1111/php.12423.
Synthesis of a disulfonated derivative of cucurbit[7]uril and investigations of its ability to solubilise insoluble drugs. Robinson, Elizabeth L.; Zavalij, Peter Y.; Isaacs, Lyle. Supramolecular Chemistry. May/Jun2015, Vol. 27 Issue 5/6, p288-297. 10p. DOI: 10.1080/10610278.2014.940952.
Macromolecular crystallography and what it can contribute to antiparasite drug discovery. Hunter, W. N.; Weiss, Manfred S. Acta Crystallographica: Section F, Structural Biology Communications. May2015, Vol. 71 Issue 5, p483-484. 2p. DOI: 10.1107/S2053230X1500789X.
Small-angle X-ray scattering analysis reveals the ATP-bound monomeric state of the ATPase domain from the homodimeric MutL endonuclease, a GHKL phosphotransferase superfamily protein. Iino, Hitoshi; Hikima, Takaaki; Nishida, Yuya; Yamamoto, Masaki; Kuramitsu, Seiki; Fukui, Kenji. Extremophiles. May2015, Vol. 19 Issue 3, p643-656. 14p. DOI: 10.1007/s00792-015-0745-2.
Micellar and biochemical properties of a propyl-ended fluorinated surfactant designed for membrane–protein study. Abla, Maher; Unger, Sebastian; Keller, Sandro; Bonneté, Françoise; Ebel, Christine; Pucci, Bernard; Breyton, Cécile; Durand, Grégory. Journal of Colloid & Interface Science. May2015, Vol. 445, p127-136. 10p. DOI: 10.1016/j.jcis.2014.12.066.
Ambiguity assessment of small-angle scattering curves from monodisperse systems. Petoukhov, Maxim V.; Svergun, Dmitri I. Acta Crystallographica: Section D. May2015, Vol. 71 Issue 5, p1051-1058. 8p. DOI: 10.1107/S1399004715002576.
Book review:
Methods of Molecular Analysis in the Life Sciences, Andreas Hofmann, Anne Simon, Tanja Grkovic and Malcolm Jones, Cambridge University Press, Cambridge, 2014, 225 pages, ISBN: 978-1107622760.
I saw a posting about this book on the CCP4 bulletin board and asked the poster to have the publisher send me a copy. The authors set out to provide a broad view of the numerous techniques available for analytical methods in the life sciences. They do a reasonably good job of providing an introduction to many of the available tools. Each chapter concludes with a section containing both references and web-based resources.
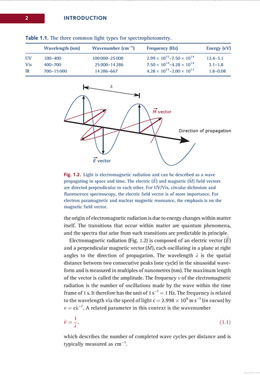
Chapter 1 reviews electromagnetic radiation and lasers. Chapter 2 covers “Spectroscopic methods” in and around the visible spectrum: atomic spectroscopy, UV/Vis spectroscopy, fluorescence spectroscopy, luminometry, circular dichroism, static and dynamic light scattering and, finally, Raman and IR spectroscopies. This is the longest and most detailed chapter in the book.
Chapter 3 reviews “Structural methods,” including electron paramagnetic and nuclear magnetic resonance, electron microscopy, X-ray crystallography, X-ray single molecule diffraction and imaging, and small angle scattering. The penultimate section on the FEL (free electron laser) is quite current and provides a good primer on the experiment.
Chapter 4 covers “Physical methods,” including centrifugation, mass spectrometry and calorimetry. The final chapter, “Surface-sensitive methods,” covers surface plasmon resonance, the quartz crystal microbalance, monolayer adsorption and atomic force microscopy.
There are some quirks in the text; for example, the authors say that the photon is “the elementary particle responsible for electromagnetic phenomena.” Then they go on to say that it “carries the electromagnetic radiation and has the properties of a wave, as well as of a particle, albeit having a mass of zero.” In my opinion this latter statement is a bit misleading – the photon is the electromagnetic radiation. Also, the formula for the calculation of R1 has extra absolute value operators that make it incorrect. Because of this error, I did a random check of ten other formulas and found no more errors. There was one other issue that I found difficult – a purplish font for the captions. Between the smaller font and lower contrast color scheme I had to squint to read the captions.
Joseph D. Ferrara, Ph.D.
Chief Science Officer
|